

 |
 |
Experiment 3-Step 2 - Taking the spectrum of each dye for identification -Step 3 - Part 1: Preparing a set of dilutions of the standard dye -Step 3 - Part 2: Measuring the absorbance of the standard solutions -Step 4 - Part 1: Determining the concentration of the unknown dye -Step 4 - Part 2: Determining if the drink exceeds the ADI guideline for Blue #1 |
Experiment 3 - Qualitative and Quantitative Analysis of Food Dyes >> Step 4 : Part 1
Experiment 3 - Qualitative and Quantitative Analysis of Food Dyes
You are now ready to prepare a 50 mL sample of your drink according to the package instructions. You will then transfer about 10mL of this solution to a vial for the colourimetric measurement. The measurement can be performed on a mixture of dyes provided the analytical wavelenghts of the two dyes present in the drink are far apart.
The instructions on the packet are: dissolve the content of the package (6g) in 2L of water.
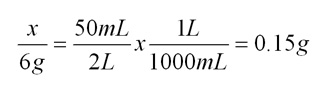
Once the drink has been prepared, the vial is measured in the colourimeter. Use the colourimeter simulation below to measure the absorbance of the unknown sample (purple vial). Once you have measured the absorbance of your sample, use your graph to determine the concentration of the solution and enter your answer into the question below the simulation. If you get stuck, tutors are included to walk you through determining the concentration of your drink sample.
0 0.00 20 0.286 40 0.546 60 0.760 80 1.028 100 1.222 Blank 20% 40% 60% 80% 100% unknown Comment Repeat Exp. BLUE #1 Now that you have measured the absorbance of your sample, use your graph to determine the concentration of the solution and enter your answer into the tutor below.
Your drink sample has the following absorbance reading:
Hint
0.727 at the analytical wavelength 620nm
Using your graph, determine the molar concentration of Blue #1 in the unknown.
(Note: please enter your answer to 2 significant figures, for very small numbers, please use scientific notation: i.e. 0.00023 can be entered as 2.3e-4)
Unknown is:
M
Good Job!
That's not quite right.
Hint:
If you are having trouble, scroll below to get step by step help in solving this problem.
The following tutors are a step by step walkthrough that show the details on how to solve the above problem.
The first task in determining the concentration is to locate the absorbance value for the unknown on the calibration graph. The calibration graph for Blue #1 is shown below. The absorbance value of 0.727 has been marked with an X.
Hint
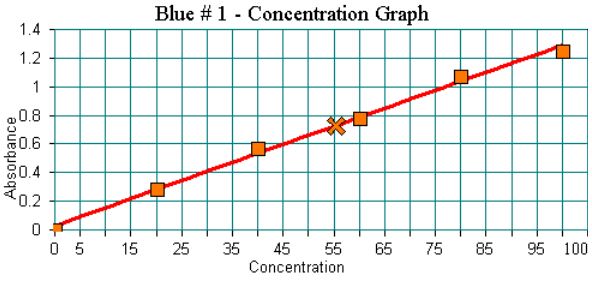 The X-axis of the graph indicates the percent concentration of the standard solution. What percent concentration on the graph correlates to this absorbance? (Please give your answer to two significant figures.)
Percent Concentration =
%
Correct!
Your answer is not correct. Please look more closely at the graph to determine the value on the X-axis that corresponds to the absorbance value marked with an X.
Hint:
Please look closely at the graph (or your own). Draw an imaginary line from the absorbance value of the unknown on the best fit line to the X-Axis. Where does it fall?
get next hint
Hint:
The absorbance value of 0.727 on the best fit line lies at about 55.4 on the X-Axis. The percent concentration of the unknown is 55%.
get previous hint
Now that we have used our calibration graph to determine the percent concentration that matches the absrobance value of the unknown, we can calculate the actual concentration that correlelates to this value. The original concentration of the Blue #1 standard solution is 4.0 x 10-6 M. Using this value, what concentration of Blue #1 corresponds to the percent concentration determined using the graph above?
Hint
(Note: please enter your answers to 2 significant figures, for very small numbers, please use scientific notation: i.e. 0.00023 can be entered as 2.3e-4)
Concentration of Unknown =
M
Good Job!
That's not quite right.
Hint:
The standard solution has a concentration of 4.0 x 10-6 M, what concentration is 55% of this value?
get next hint
Hint:
The percent concentration of the unknown is 55%. Multiplying this value times the original concentration of the standard solution will yield the concentration of the unknown. What is this concentration?
get previous hint
get next hint
Hint:
The concentration of the unknown is 0.55 x 4.0 x 10-6 = 2.2 x 10-6 M
get previous hint
|