

 |
 |
Experiment 3-Step 2 - Taking the spectrum of each dye for identification -Step 3 - Part 1: Preparing a set of dilutions of the standard dye -Step 3 - Part 2: Measuring the absorbance of the standard solutions -Step 4 - Part 1: Determining the concentration of the unknown dye -Step 4 - Part 2: Determining if the drink exceeds the ADI guideline for Blue #1 |
Experiment 3 - Qualitative and Quantitative Analysis of Food Dyes >> Step 3 : Part 1
Experiment 3 - Qualitative and Quantitative Analysis of Food Dyes
Preparing Dilutions:
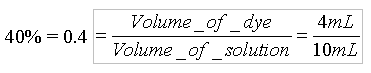
Each standard dye available in the lab has a label with its concentration. Because the standard dyes have different starting concentrations, the diluted solutions will have different molarities based upon their original concentrations. If the concentration of the Blue #1 standard is 4.0x10-6 M, then the molar concentration of the 40% diluted solution would be: Concentration of the diluted solution = 0.4 * 4.0x10-6 M = 1.6 x 10-6 M Use the above calculations as a guide to determine the volumes of water and dye needed to create the diluted solutions in the table below. Once you have entered the volumes required, determine the concentrations (molarities) of your diluted solutions. If you get stuck, you can click the (hint) button for an explanation of any calculation.
Please complete the table below:
Hint
(Note: please enter your answers to 2 significant figures, for very small numbers, please use scientific notation: i.e. 0.00023 can be entered as 2.3e-4)
Sample
%Conc
Vol. Dye
Vol. Water
Conc (M) Dye(Blue #1)
Water Blank
0
0
10
0
Standard 1
100%
mL
mL
4.0 x 10-6M Standard 2
80%
mL
mL
M
Standard 3
60%
mL
mL
M
Standard 4
40%
4.0 mL
6.0 mL
1.6 x 10-6M
Standard 5
20%
mL
mL
M
Your answer is correct.
Your answer is incorrect, please check your calculation again. If you are stuck, please click the 'hint' button.
Hint:
Standard solution #1 should have a 100% concentration of dye. In a 10 mL solution, how much dye would this be?
get next hint
Hint:
Because the concentration of dye is 100%, all 10.0 mL of the 10.0 mL vial will be dye.
get previous hint
Hint:
Standard solution #1 should have a 100% concentration of dye. In the 10 mL vial, 10 mL of the volume is dye. How much of the solution should be water?
get next hint
Hint:
Because the concentration of dye is 100%, there is no water added. (0.0 mL)
get previous hint
Hint:
Standard solution #2 should have an 80% concentration of dye. In a 10 mL solution, how much dye would this be?
get next hint
Hint:
Because the concentration of dye is 80%, the 10 mL of the 10 mL vial contains 0.8 * 10 mL dye = 8.0 mL dye. You can also conceptually consider this dilution as 8 parts dye and 2 parts water.
get previous hint
Hint:
In the dye volume calculation, we see that an 80% solution resulted in 8.0 mL of dye being added to the vial. If the total volume is 10 mL, how much of the solution should be water?
get next hint
Hint:
In the 80% soution, the 10 mL volume is comprised of 8.0 mL of dye; the remaining 2.0 mL will be made up of water.
get previous hint
Hint:
Standard solution #2 should have an 80% concentration of dye. The original concentration of the dye solution was 4.0 x 10-6 M, what is 80% of this amount?
get next hint
Hint:
Multiplying the original concentration by 80% yields the concentration of the diluted solution. (4.0 x 10-6 M * 0.8 = 3.2e-6). The concentration of the 80% solution is 3.2e-6 M dye.
get previous hint
Hint:
Standard solution #3 should have a 60% concentration of dye. In a 10 mL solution, how much dye would this be?
get next hint
Hint:
Because the concentration of dye is 60%, the 10 mL of the 10 mL vial contains 0.6 * 10 mL dye = 6.0 mL dye. You can also conceptually consider this dilution as 6 parts dye and 4 parts water.
get previous hint
Hint:
In the dye volume calculation, we see that a 60% solution resulted in 6.0 mL of dye being added to the vial. If the total volume is 10 mL, how much of the solution should be water?
get next hint
Hint:
In the 60% soution, the 10 mL volume is comprised of 6.0 mL of dye; the remaining 4.0 mL will be made up of water.
get previous hint
Hint:
Standard solution #3 should have a 60% concentration of dye. The original concentration of the dye solution was 4.0 x 10-6 M, what is 60% of this amount?
get next hint
Hint:
Multiplying the original concentration by 60% yields the concentration of the diluted solution. (4.0 x 10-6 M * 0.6 = 2.4e-6). The concentration of the 60% solution is 2.4e-6 M dye.
get previous hint
Hint:
Standard solution #5 should have a 20% concentration of dye. In a 10 mL solution, how much dye would this be?
get next hint
Hint:
Because the concentration of dye is 20%, the 10 mL vial should contain 0.2 * 10 mL dye = 2.0 mL dye. You can also conceptually consider this dilution as 2 parts dye and 8 parts water.
get previous hint
Hint:
In the dye volume calculation, we see that a 20% solution resulted in 2.0 mL of dye being added to the vial. If the total volume is 10 mL, how much of the solution should be water?
get next hint
Hint:
In the 20% soution, the 10 mL volume is comprised of 2.0 mL of dye; the remaining 8.0 mL will be made up of water.
get previous hint
Hint:
Standard solution #5 should have a 20% concentration of dye. The original concentration of the dye solution was 4.0 x 10-6 M, what is 20% of this amount?
get next hint
Hint:
Multiplying the original concentration by 20% yields the concentration of the diluted solution. (4.0 x 10-6 M * 0.2 = 8.0e-7). The concentration of the 20% solution is 8.0e-7 M dye.
get previous hint
|