

 |
 |
Experiment 3-Step 2 - Taking the spectrum of each dye for identification -Step 3 - Part 1: Preparing a set of dilutions of the standard dye -Step 3 - Part 2: Measuring the absorbance of the standard solutions -Step 4 - Part 1: Determining the concentration of the unknown dye -Step 4 - Part 2: Determining if the drink exceeds the ADI guideline for Blue #1 |
Experiment 3 - Qualitative and Quantitative Analysis of Food Dyes >> Introduction
Experiment 3 - Qualitative and Quantitative Analysis of Food DyesIntroductionIn this experiment the goal is to determine the amount of dyes present in a powdered beverage in order to examine the allegation that the manufacturer is exceeding the allowable amount of the artificial food dyes in the drinks it produces. The drink may contain more than one dye, and so the dyes must first be separated by column chomatography and then identified by colourimetric analysis. This is a qualitative analysis of the dyes. Once the dyes have been identified we can determine their concentration in the prepared drink using Beer's Law, another colourimetric technique. This is a quantitative analysis of the dyes. The experimental strategy is shown schematically below: Qualitative Analysis: Step 1 and Step 2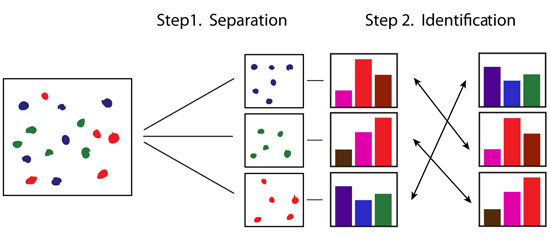 Quantitative Analysis: Step 3 and Step 4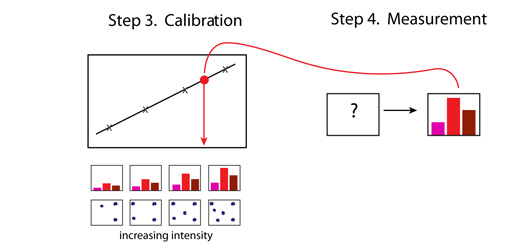 We start with a packet of drink that contains two different dyes. For example, a grape drink may contain a red and a blue dye that blend to result in a purple colour drink. Our goal is to identify each of the dyes, and then, once identified, we can measure the amount of each. We express the amount as a concentration of the dye in the drink when it is prepared according to the package instructions.
Action: We dissolve a few grains of the solid drink in about a mL of water. We pour this mixture on a chromatographic column and wash it down with a lot of water. Result: The dyes leave the column at different times and we collect them in separate vials. Action: We take a spectrum of each dye, and also a spectrum of each similarly coloured standard dye available in the laboratory. Result: Each dye has a unique spectral pattern. By matching the unknown dye to one of the standards we uncover its identity. Action: We prepare a set of dilutions of the standard dye (its concentration is written on the bottle) that matched our unknown. We measure the absorbance of each diluted solution at the analytical wavelength. Result: We obtain a calibration plot of absorbance versus concentration at the analytical wavelength for the standard dye. We can use this plot to determine the concentration of the unknown once we measure its absorbance. Action: We prepare the drink according to package instructions and measure its absorbance at the analytical wavelength for the calibrated dye. Result: We can read the concentration of the dye from the calibration plot. We can now calculate the amount of the dye (in mg) per L of the drink and determine the ADI value. The following pages contain tutors to assist you with identification of the dyes and the calculations required in some of the above steps.
|