How Buffers Work
How does a mixture of a weak acid and its conjugate base help buffer a solution against pH changes?
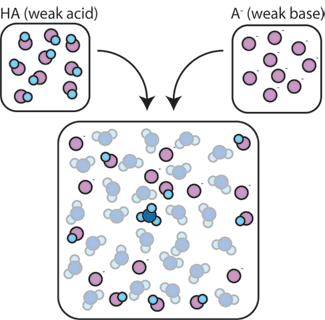
If we mix a weak acid (HA) with its conjugate base (A-), both the acid and base components remain present in the solution. This is because they do not undergo any reactions that significantly alter their concentrations. The acid and conjugate base may react with one another, HA + A- → A- + HA, but when they do so, they simply trade places and the concentrations [HA] and [A-] do not change. In addition, HA and A- only rarely react with water. By definition, a weak acid is one that only rarely dissociates in water (that is, only rarely will the acid lose its proton H+ to water). Likewise, since the conjugate base A- is a weak base, it rarely steals a proton H+ from water.
So, the weak acid and weak base remain in the solution with high concentrations since they only rarely react with the water. However, they are very likely to react with any added strong base or strong acid.
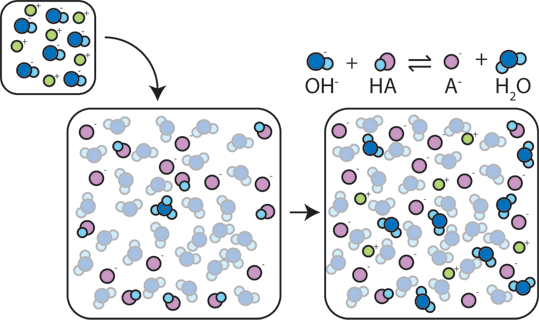
If a strong base is added to a buffer, the weak acid will give up its H+ in order to transform the base (OH-) into water (H2O) and the conjugate base: HA + OH- → A- + H2O. Since the added OH- is consumed by this reaction, the pH will change only slightly.
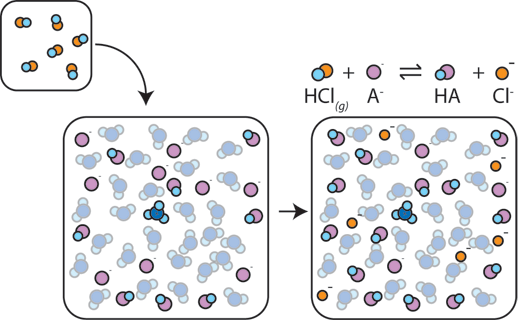
If a strong acid is added to a buffer, the weak base will react with the H+ from the strong acid to form the weak acid HA: H+ + A- → HA. The H+ gets absorbed by the A- instead of reacting with water to form H3O+ (H+), so the pH changes only slightly.
See the next page for a tutor that leads you through the calculations involved in making a buffer.