pH in the Absence of a Buffer
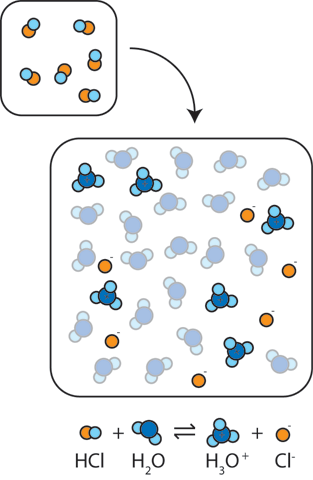
If we add a strong acid or strong base to water, the pH will change dramatically. For instance, adding a strong acid such as HCl to water results in the reaction HCl + H2O → H3O+ + Cl-. In other words, the proton (H+) from the acid binds to neutral water molecules to form H3O+ raising the concentration of H+. The resulting large concentration of (H+) makes the solution more acidic and leads to a dramatic drop in the pH.
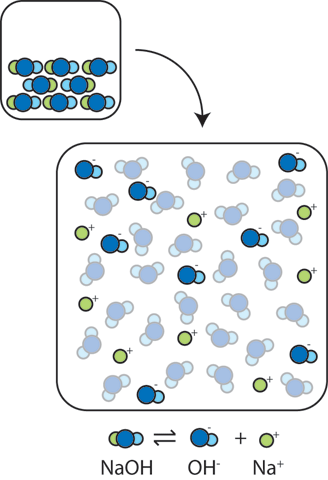
Solid NaOH consists of Na+ and OH- ions packed into a crystalline lattice. When this solid is added to water, the ions float apart leading to extra OH- ions in the water: NaOH → OH- + Na+. The resulting large concentration of OH- makes the solution more basic and leads to a dramatic increase in the pH. (Remember that since the product of concentrations, [OH-][H+], remains fixed at Kw=10-14, as the concentration of OH- ions goes up, the concentration of H+ ions goes down.)