Buffer Capacity
What factors determine the effectiveness (or capacity) of a buffer?
The goal of a buffer is to keep the pH of a solution within a narrow range. While the ratio of [A-]/[HA] influences the pH of a solution, the actual concentrations of A- and HA influence the effectiveness of a buffer.
The more A- and HA molecules available, the less of an effect addition of a strong acid or base will have on the pH of a system. Consider the addition of a strong acid such as HCl. Initially, the HCl donates its proton to the weak base (A-)through the reaction A- + HCl → HA + Cl-. This changes the pH by lowering the ratio [A-]/[HA], but as long as there is still a lot of A- present, the change in pH will be small. But if we keep adding HCl, the weak base A- will eventually run out. Once the A- is gone, any additional HCl will donate its proton to water (HCl + H2O → H3O+ + Cl-). This will dramatically increase the concentration [H+] and so the pH drops.
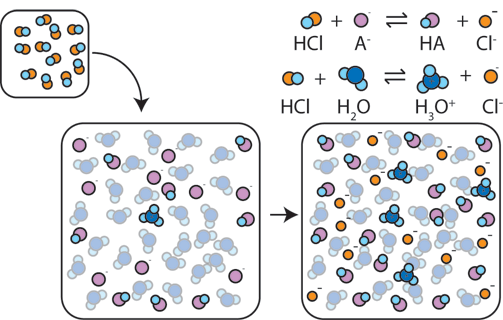
We call this "breaking the buffer solution", and we call the amount of acid a buffer can absorb before it breaks the "buffer capacity for addition of strong acid". A solution with more weak base, [A-], has a higher buffer capacity for addition of strong acid.
Similarly, a buffer will break when the amount of strong base added is so large it consumes all the weak acid, through the reaction HA + OH- → A-+ H2O. A solution with more weak acid, [HA], has a higher buffer capacity for addition of strong base.
So although the pH of a buffer is determined by only the ratio [A-]/[HA], the ability of the buffer to absorb strong acid or base is determined by the individual concentrations of [A-] and [HA].
For additional practice with the concept of buffer capacity, please try the following tutor.
See the next two pages for more practice problems.