

 |
 |
Analysis of Food Dyes-Step 2 - Taking the spectrum of each dye for identification -Step 3 - Part 1: Preparing a set of dilutions of the standard dye -Step 3 - Part 2: Measuring the absorbance of the standard solutions -Step 4 - Part 1: Determining the concentration of the unknown dye -Step 4 - Part 2: Determining if the drink exceeds the ADI guideline for Blue #1 |
Qualitative and Quantitative Analysis of Food Dyes >> Step 3 : Part 1
Qualitative and Quantitative Analysis of Food Dyes
Preparing Dilutions:
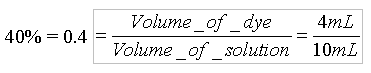
Each standard dye available in the lab has a label with its concentration. Because the standard dyes have different starting concentrations, the diluted solutions will have different molarities based upon their original concentrations. If the concentration of the Blue #1 standard is 4.0x10-6 M, then the molar concentration of the 40% diluted solution would be: Concentration of the diluted solution = 0.4 * 4.0x10-6 M = 1.6 x 10-6 M Use the above calculations as a guide to determine the volumes of water and dye needed to create the diluted solutions in the table below. Once you have entered the volumes required, determine the concentrations (molarities) of your diluted solutions. If you get stuck, you can click the (hint) button for an explanation of any calculation.
|