Thermodynamics Concept Test
Problem 1
A piston is connected to a drive train that causes a car to move. The flame below is turned on, which heats up
the gas causing it to expand. 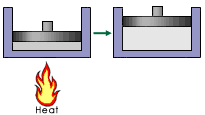 The
signs of q and w for the gas in the cylinder are:
The
signs of q and w for the gas in the cylinder are:
- q + w +
- q + w -
- q - w +
- q - w -
Problem 2
Consider burning acetylene:
C2H2(g) + 3/2O2(g) <---> CO2 (g) + H2O(l) ΔH = -1301.1 kJ
What is ΔH for the reaction:
2CO2(g) + 2H2O(l) <---> 2C2H2(g) + 3O2(g)
- ΔH = -2602.2 kJ
- ΔH = -1301.1 kJ
- ΔH = 1301.1 kJ
- ΔH = 2602.2 kJ