Problem Description:
You are trying to explain two chemistry concepts to a fellow student. You will prove that the law of mass conservation is correct. You will also show your fellow student how to write molecular, total ionic, and net ionic equations. These equations will indicate to your fellow student how ionic substances behave in water.
Lab Description:
Reactions between ionic species may lead to a "double displacement reaction" (a/k/a metathesis). In an aqueous environment, one knows a reaction has occurred if a precipitate (a solid), a liquid (usually water), or a gas forms.
A Generic Double Displacement Reaction has the form:
AX (aq) + BY (aq) → AY + BX (s, l, or g)
To decide whether a reaction has occurred, inspect each reactant species. If a reaction occurs a new combination of cation and anions must occur.
A2+ B2+ X2- Y2-
The only possible re-combinations are AY and BX. (You always have a cation and an anion in an ionic compound.)
After evaluating possible recombination of the ions, evaluate whether AY or BX will form a molecular substance (solid, liquid, or gas) or whether they will remain isolated ions in the solution. In the following exercise, we will limit the analysis to the formation of a solid, called a precipitate ("ppt").
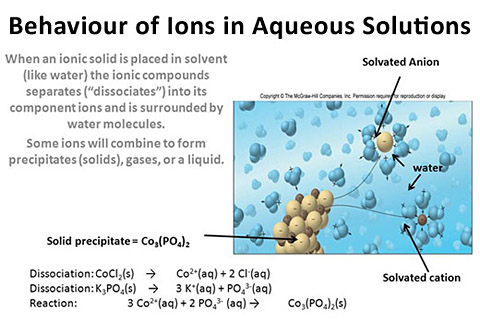
"Soluble ionic compound" means that when a solid compound is placed in water, the ions of the compound are separated and isolated by water molecules. The polar water molecules help offset the charge of the ions. These ions do not re-form with other soluble ions to form a new compound.
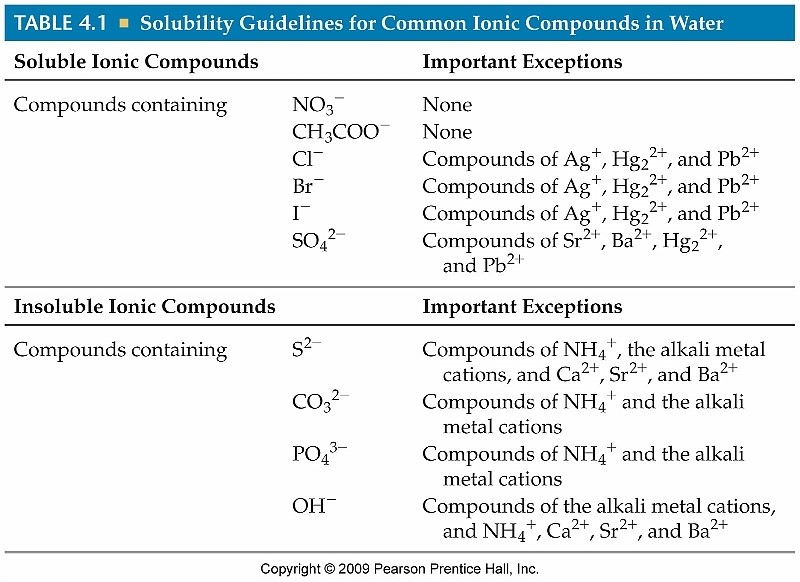
A reaction will occur between ions in an aqueous (water) environment if two "insoluble" ions are combined though. Use the following solubility table to guide your decisions what type of solid is formed.
Consider the following reaction:
Na2CO3 (aq) + CaCl2 (aq) → CaCO3 (s) + 2 Na+ (aq) + 2 Cl- (aq)
The soluble Na2CO3 will dissociate into the ions Na+ and CO32- in water. Likewise, CaCl2 forms Ca2+ and Cl- ions.
Na+ Ca2+ CO32- Cl-
Possible re-combinations:
CaCO3 and NaCl
Solid CaCO3 will form in the solution but Na+ and Cl- will remain hydrated ions and have no tendency to reform.
Experimental Procedure:
Procedure 1: Prove the Law of Mass Conservation
The Law of Conservation of Matter states that matter can neither be created nor destroyed.
Place the Erhlenmeyer flasks with 1.00 g AgNO3 and 1.00 g NaCl on the benchtop. Place the distilled water on the benchtop.
The solution labeled "1.00 g NaCl" contains 1.00 g of NaCl dissolved in water. Use the solution information window to determine the molarity of Na+ and Cl- and confirm that these add to 1.00 g. For example, to calculate the grams of Na+ in the solution: 0.170529 M x 0.100 L x 22.99 g/mol = 0.392 g Na+. (Recall that Molarity (M) has units of mol/L.)
The flask labeled "1.00 g AgNO3" contains 1.00 g of solid AgNO3. Add 100.0 mL of water to this flask by choosing the "Precise Transfer" mode, input 100.0 mL in the "Transfer Amount" and then "Pour" it into the flask. Use the solution information window to determine the Molarity of Ag+ and NO3- and convert to the number of grams of Ag+ and NO3- in the solution.
Clear the flasks off the benchtop.
Procedure 2
Place a new 1.00 g NaCl Erhlenmeyer flask and the solid AgNO3 on the benchtop.
Add 1.00 g of solid AgNO3 from the bottle of AgNO3 to the 1.00 g solution of NaCl on your workbench. To do this, choose the "Precise Transfer" mode in your Tools icon. Place the bottle of solid AgNO3 over the solution of NaCl and input 1.00 gram into the "Transfer Amount." "Pour" it into the solution.
Written Assignment:
Procedure 1
Identify the mass of each reactant ion and the total mass of the reactants.
Identify the mass of each product and the total mass of the product.
Explain how this experiment confirms the Law of Mass Conservation.
Procedure 2
Write a balanced molecular equation for the reaction of solid AgNO3 with aqueous NaCl. Be sure to include the correct number of coefficients and the state of the species (aq, s, l or g).
Write the total ionic equation for this reaction, including all physical states.
Write the net ionic equation for this reaction, including all physical states.
Identify the spectator ions.
If the chloride ion is usually soluble, how can solid AgCl form? (Be sure to discuss the exception to the chloride rule.)
Problems:
Using the Solubility Rules, write the balanced molecular, total ionic, and net ionic equations (including physical states) for the following:
Hints: There is no such thing as Na2+ or Na3+. Na is in group 1A, so you know it can only form a +1 charge. (For example, it will form 3 Na+ ions for each Na3PO4.) There is a diatomic molecule Cl2. But when ionic compounds are dissolved in water, they form ions so the dissociation of MgCl2 is to an Mg2+ and 2 Cl- ions, as an example.
Na2S (aq) + CuCl (aq) →
CoCl2 (aq) + KOH (aq) →